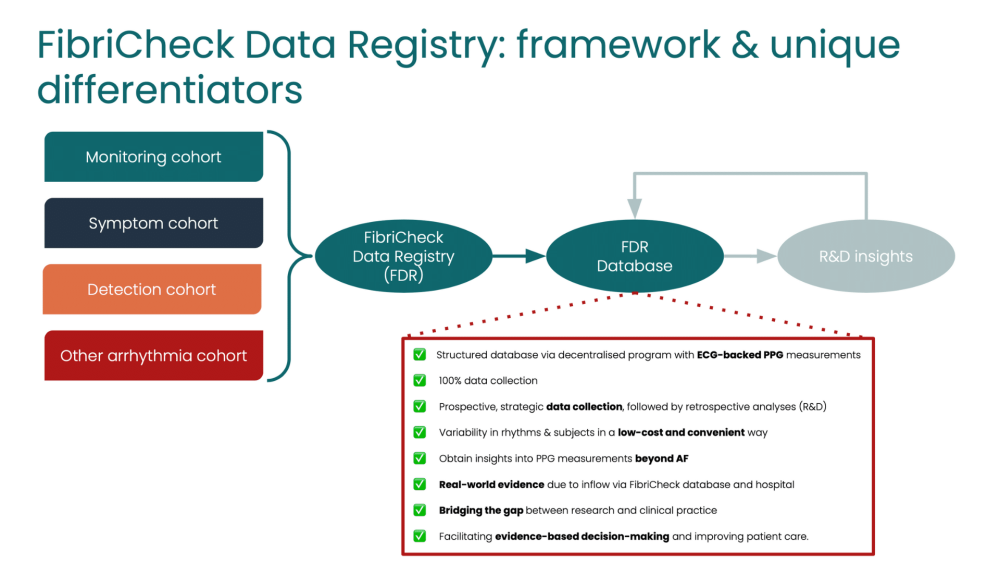
At FibriCheck, we believe that the best way to improve our services to our users and to make advancements in a fast-paced world is by never ceasing to explore new opportunities and by finding innovative ways to get the most out of the potential of real-world evidence in free-living conditions. In light of this, we created the FibriCheck Data Registry (NCT06282393), a unique approach to collecting high quality, real-world data that enables us to continuously evaluate our performance and fuel our research and development activities.
What is the FibriCheck Data Registry?
Today, the gold standard in heart rhythm monitoring is electrocardiography (ECG), but more and more evidence is available regarding the untapped potential of photoplethysmography (PPG) for cardiac monitoring. With the help of artificial intelligence (AI), we can further unlock this potential, but for that, we need real-world data from a broad range of individuals in free-living conditions. The FibriCheck Data Registry is a unique strategy to collect this data. Real-world data allows us to continuously monitor our product performance. More importantly, it supports our scientific advancements, the development, and improvement of upcoming AI models which significantly increases the capabilities and robustness of our technology.
In recent academic studies, independent researchers have demonstrated the breakthroughs of our algorithm and AI model by maxing out FibriCheck’s clinical performance data to detect or rule-out atrial fibrillation, and reducing our insufficient signal quality levels. When compared to other state-of-the-art-technologies, our algorithm and AI model have a higher performance in detecting atrial fibrillation, and a lower prevalence of bad quality recordings. (as presented by Femke Wouters at the Heart Rhythm Society event – comparative evaluation of digital consumer devices for atrial fibrillation detection: a validation study)
We are very proud of this achievement, but in order to continue to grow, we aim to evaluate and stress-test our capabilities beyond controlled hospital-environments. With the FibriCheck Data Registry, we want to bring additional value to our users and healthcare providers by:
- Developing new models that help us identify other heart rhythm disorders, and further explore potential indications for use beyond atrial fibrillation.
- Creating a structured, synchronized ECG-PPG data library that educates and supports healthcare providers in their clinical decision making
This is where the FibriCheck Data Registry comes in. By leveraging real-world PPG data and comparing it to ECG recordings, we want to standardize PPG and expand our capabilities to recognize more heart rhythm conditions. These heart rhythm conditions are currently visible on ECG. We believe that a large portion of these conditions can be seen on PPG as well, they are just not recognizable yet. In order to recognize them, a very structured and simultaneous collection of ECG and synchronized PPG results from users is key.
How does the FibriCheck Data Registry work?
We intend to collect real-world data by asking both users with irregular measurement results and healthy volunteers to perform PPG measurements on their smartphone, in combination with simultaneous ECG measurements as a gold standard reference.
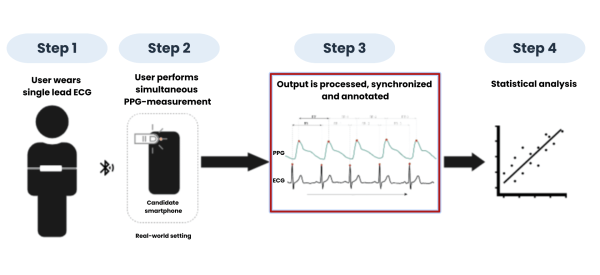
By synchronizing the data to the heartbeat, we can create a deep understanding of patient conditions and increase the richness of our capabilities. With our database of over 1 million users and the easy scalability of this project, we can offset new innovation and broaden our scope to include several other heart rhythm disorders which are currently not yet recognized to be identifiable using PPG.
This way of working is a unique, powerful, and cost-efficient addition to clinical studies, as we can learn from real-world settings instead of a controlled clinical environment. This allows for more richness in our data and more possibilities to learn and develop. As we can control the logistics of the Data Registry ourselves, we can easily enrich our dataset continuously and use it to further develop our technology.
What is the project setup?
We aim to include data from a broad population of users and patients from different backgrounds. We have clinical sites that refer patients for enrollment, and we have our consumer population which we can use to invite people to participate in the Registry in case specific heart rhythm disorders are identified.
People participating in the FibriCheck Data Registry receive clear instructions of the study and the expectations. Once they give their consent, they can be enrolled in the study. After a brief questionnaire about their demographics, they receive an ECG sensor that connects to the FibriCheck application. Test subjects are asked to wear this device for a period of 2 weeks, and perform FibriCheck measurements according to a predefined protocol. After 2 weeks, they send the ECG sensor back, after which all data is analyzed and handled with utmost care and GDPR compliance.
The FibriCheck Data Registry can make a meaningful change for our users. FibriCheck is already the trusted partner in heart health for over a million people worldwide because they experience symptoms or have concerns about their heart health. By studying divergent data from our users compared to golden standard ECG measurements, we can improve our services and provide these people with additional answers regarding their heart health.
With this Registry, we want to make a positive impact on clinical routine and clinical decision making by offering insights into various arrhythmias and their interpretation based on synchronized ECG-PPG measurements. We also want to continue building trust and credibility with healthcare providers and support the growing acceptance of PPG-technology to welcome it into routine clinical care.
Put your PPG interpretation skills to the test and learn more about the value of the FibriCheck Data Registry: https://bit.ly/3YVSDwJ
Contact us for more information
Created on June 5th, 2024 at 01:33 pm
Last updated on June 28th, 2024 at 10:28 am