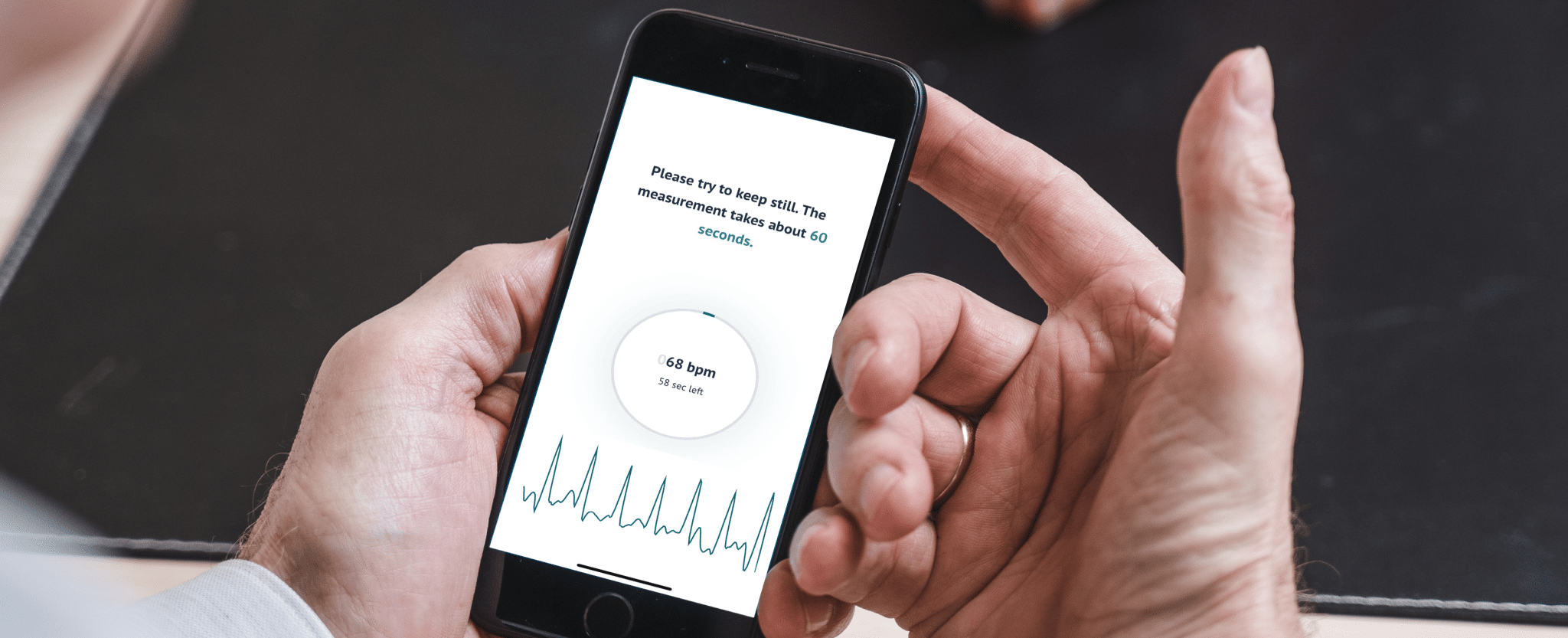
FibriCheck’s 2025 wrap-up
With 2025 coming to an end, we’re proud to look back on a year defined by major progress. With significant product improvements, powerful clinical validation, and new partnerships, we continued advancing our mission to make reliable heart health measurements accessible…