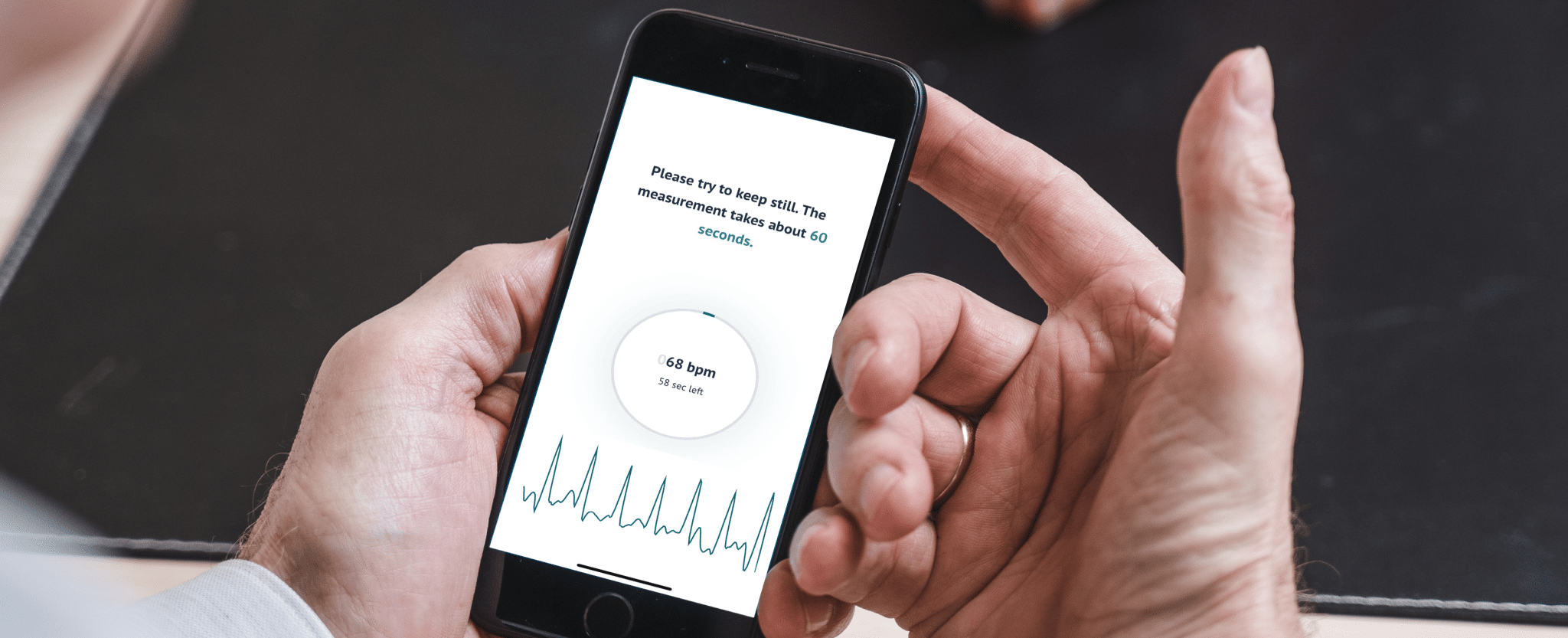
A world first – FibriCheck app automatically detects both silent and intermittent atrial fibrillation at night, dramatically reducing the risk of stroke in 40-plussers
Hasselt, Belgium, 31 March 2020 – FibriCheck, the first CE- and FDA-approved medical app, today announces another world premiere: the first smartwatch app that automatically monitors users’ heart rhythms during their sleep. The app, which can be integrated into any brand of smartwatch, detects episodes of both intermittent and silent atrial fibrillation (AF), where symptoms are only felt on and off or not at all1, making diagnosis extremely difficult. FibriCheck’s latest innovation offers an easy and effective solution for detecting the most common form of heart rhythm disorder which is responsible for 1 in 4 strokes². As such, it represents a unique opportunity for smartwatch manufacturers to add genuine value to their products in the form of potentially life-saving, built-in technology.

Until now, atrial fibrillation could only be detected via expensive medical examinations in a clinical setting. Which is why many people do not have themselves tested, despite the relatively high risk: 1 in 4 people over the age of 40 will develop the condition in their lifetime4.
Lars Grieten, CEO FibriCheck: “Our goal is to make it as easy as possible for people to know if they have a heart rhythm irregularity, to prevent AF strokes from happening. The FibriCheck heart rhythm app is a device-agnostic medical software application that can easily be integrated into any phone or wearable product that uses PPG (photoplethysmography) sensors. For smartphone, smartwatch and consumer technology companies, this opens up new possibilities to expand product offerings and provide consumers with innovative ways to manage their health and health-related conditions, including better heart health.”
Extensively validated in clinical settings

Fully continuous monitoring
FibriCheck was selected as a high-impact innovation by the 2020 NHS Innovation Accelerator (NIA) programme, another step in commercially expanding and scaling up FibriCheck’s market reach. One of the company’s upcoming projects includes making fully continuous monitoring a reality. In this not-too-distant scenario, users will be able to have their heart rhythm monitored constantly and automatically, even during the day.
Lars Grieten, CEO FibriCheck: “This is the first step in turning consumer devices into fully automated medical diagnostic devices. Our next steps will be to add even more devices, and more frequent monitoring, to improve the user experience and make it easier to deliver better capabilities supporting heart health.”
References
- Xiong, Proietti, Senoo, Lip. Asymptomatic versus symptomatic atrial fibrillation: A systematic review of age/gender differences and cardiovascular outcomes. PubMed – NCBI. Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/25974193 Int J Cardiol. 2015 Jul 15;191:172-7. doi: 10.1016/j.ijcard.2015.05.011. Epub 2015 May 7. Comparative Study; Review; Systematic Review
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D et al. (2016) 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37 (38): 2893-2962.Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD et al. (2012) Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation 125 (23): 2933-2943.
- Understanding Risks of Stroke and Blood Thinners, CardioSmart, American College of Cardiology, May 2017, retrieved from: https://www.cardiosmart.org/Heart-Conditions/Atrial-Fibrillation/Content/Risks-of-Stroke-and-Blood-Thinners
- Laila Staerk et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018;361:k1453 | doi: 10.1136/bmj.k1453, retrieved from: https://ahajournals.org/doi/full/10.1161/01.CIR.0000140263.20897.42
Created on March 30th, 2020 at 07:51 pm
Last updated on January 6th, 2023 at 05:25 pm